The binding energies of hydrogen, oxygen, water and peroxide molecules... | Download Scientific Diagram

Hydrogen nucleus combines to form helium then energy is released. Binding energy/nucleon of `He` is - YouTube

In a hydrogen atom, the binding energy of the electron in the ground state is `E_(1)` then - YouTube
If the binding energy of the electron in a hydrogen atom is 13.6 eV, - Sarthaks eConnect | Largest Online Education Community
If the binding energy of the electron in the ground state of hydrogen atom is E, then the frequency of electron in the nth orbit is
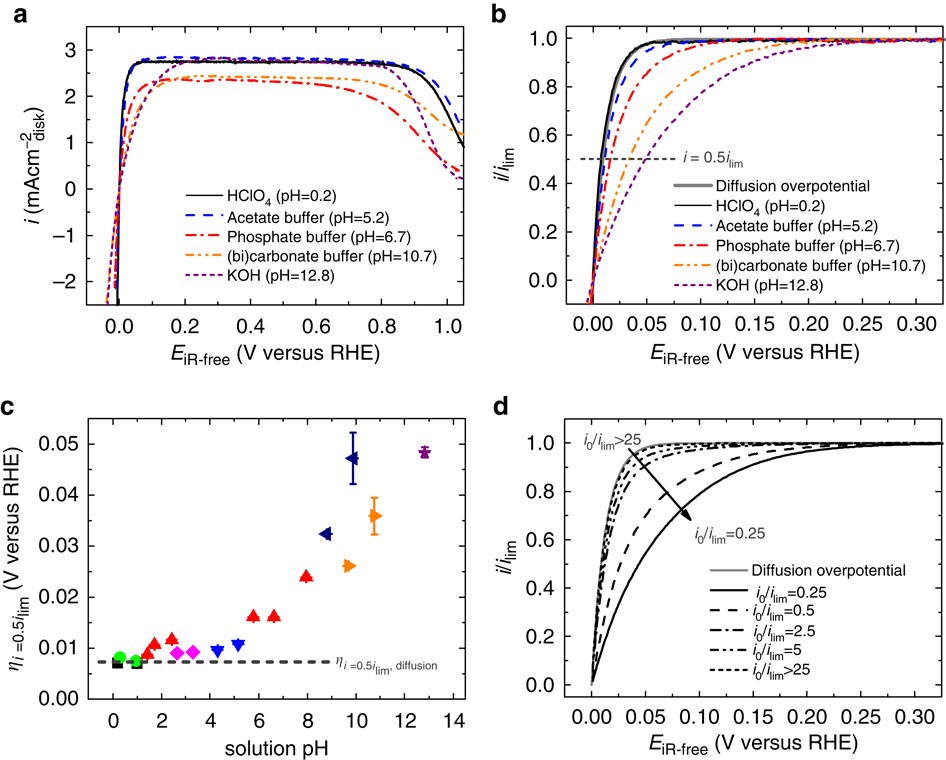
Correlating hydrogen oxidation and evolution activity on platinum at different pH with measured hydrogen binding energy | Nature Communications

A hydrogen atom in a state having a binding energy of 0.85 eV makes transition to a state with - YouTube

Color online) Binding energy per atom of the onedimensional hydrogen... | Download Scientific Diagram

If the binding energy of 2nd excited state of hydrogen like sample is 24ev approx,then determine the atomic number of - Chemistry - Structure of Atom - 12877143 | Meritnation.com

If the binding energy of the electron in a hydrogen atom is 13.6 eV, the energy required to remove the electron from the first excited state of Li^(++) is

The binding energy of `e^(-)` in ground state of hydrogen atom is 13.6 eV . The energies required to - YouTube

In a hydrogen atom, the binding energy of the electron in the nth state is En ,then the frequency of revolution of the electron in the nth orbit is:

The hydrogen binding energy (HB) for all suggested base pairs using... | Download Scientific Diagram
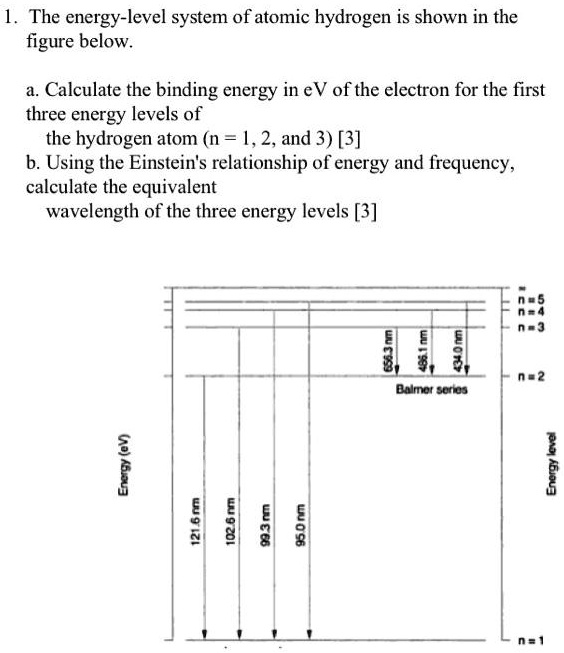
SOLVED: The energy-level system of atomic hydrogen is shown in the figure below. Calculate the binding energy in eV of the electron for the first three energy levels of the hydrogen atom (